Chapter 4 Somatic Variant Classification Guidelines
4.1 Learning Objectives
This chapter will cover:
- AMP/ASCO/CAP Guidelines
- ClinGen/CGC/VICC Oncogenicity SOP
4.2 AMP/ASCO/CAP Guidelines
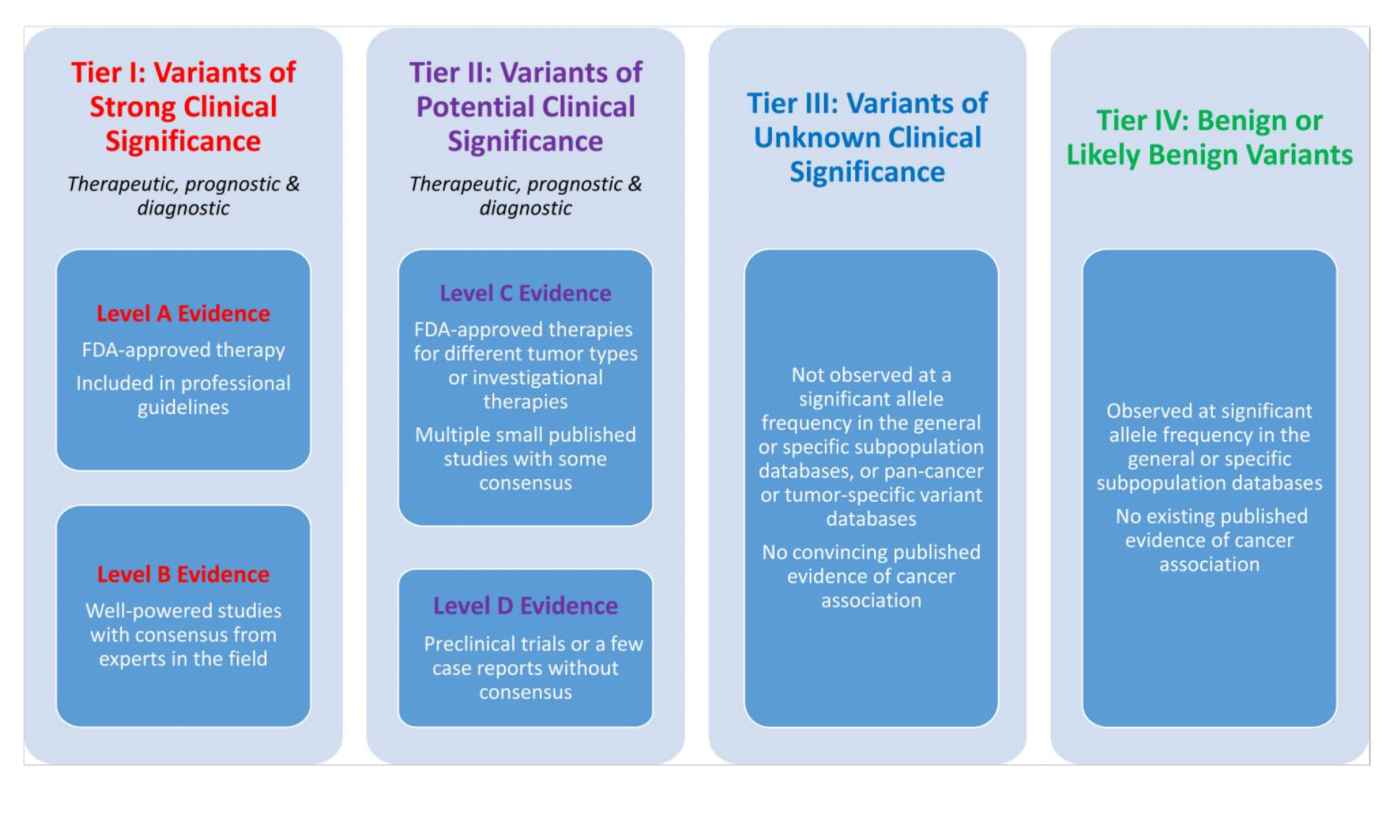
Guidelines for the interpretation of clinically actionable somatic variants have been established by AMP/ASCO/CAP (Li et al. 2017), ESMO (Mateo et al. 2018) and others. The AMP/ASCO/CAP guidelines were published in 2017, with the aim of standardizing the interpretation and reporting of molecular results among clinical laboratories. These guidelines focus on the Predictive/Therapeutic, Diagnostic, and Prognostic evidence categories discussed above. The guidelines introduce a system of tiering somatic variants based on clinical knowledge associated to the variant. The tiering system considers the state of knowledge and clinical actionability of a somatic variant within a specific cancer type, along with an associated drug if applicable. Briefly, Tier I Level A variants are established in guidelines such as those provided by the FDA or WHO. For example, in the presence of the EGFR L858R variant, the tyrosine kinase inhibitor erlotinib is approved for use in non small cell lung cancer, and therefore in this context the variant is Tier I Level A. Tier I Level B variants are those where a reasonable amount of knowledge is present for the variant and disease in the form of clinical trials, but approvals are not in place. If regulatory approvals become established, then the variant will be upgraded to Tier I Level A. Tier II Level C variants are those where the state of knowledge has not proceeded beyond case studies. This Tier also applies to cases where a drug which is established for targeted use against a given variant in a certain cancer type is applied against the same variant but in a different cancer type. Tier II Level B variants are those where studies have not yet progressed beyond preclinical work. Note that besides targetable driver variants, these tiers can also be applied to resistance variants, as this variant type also appears in guidelines.
4.3 ClinGen/CGC/VICC
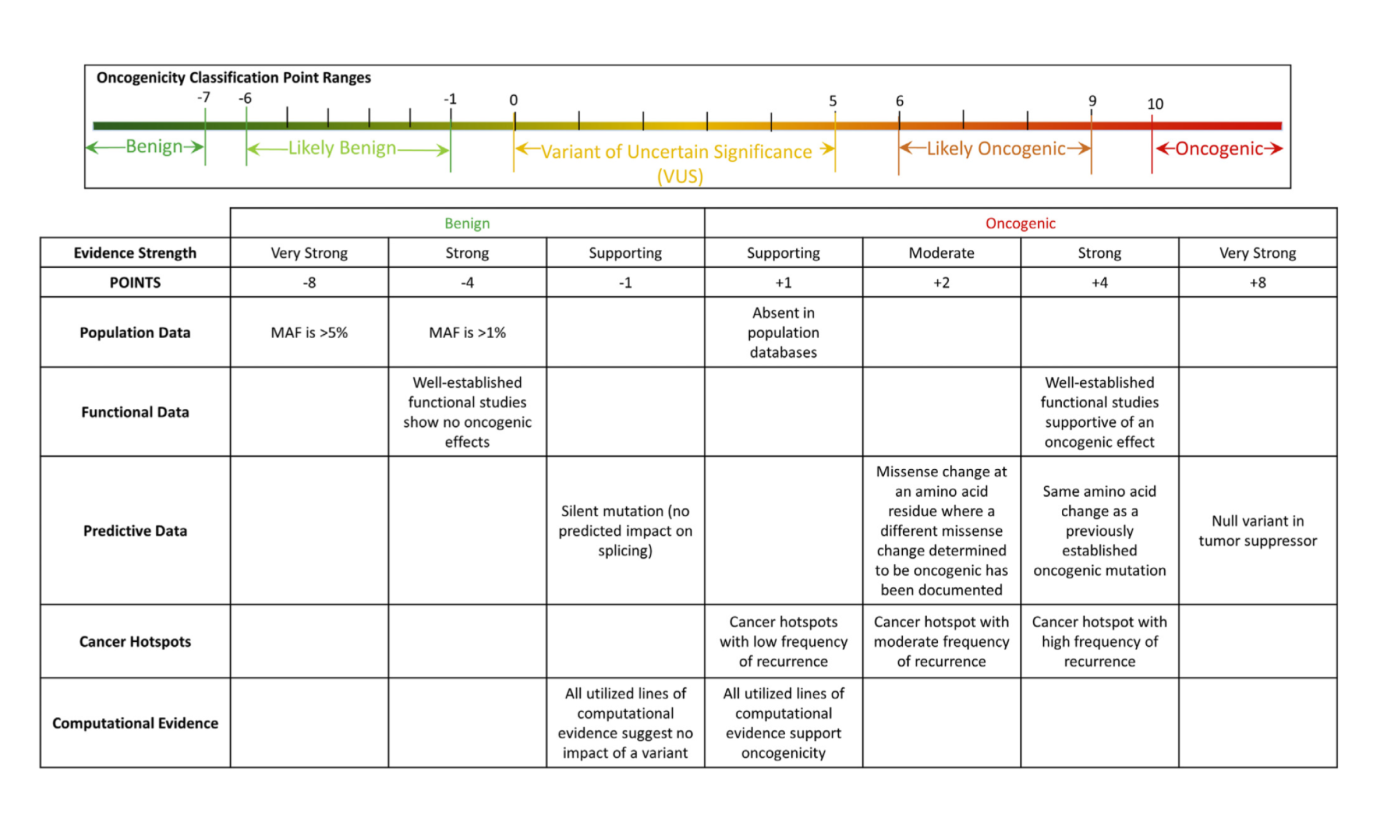
The ClinGen/CGC/VICC guidelines were published in 2022 (Horak et al. 2022). While earlier guidelines focused on predictive, diagnostic, and prognostic evidence for a variant, the guidelines proposed by ClinGen/CGC/VICC provide a direct, systematic, and comprehensive set of standards and rules to classify the oncogenicity of a somatic variant in a quantitative manner. These guidelines provide a system of codes, each carrying a certain numeric weight. Codes are applied when certain criteria are met, such as preclinical assays demonstrating an oncogenic effect (or lack of an oncogenic effect in the presence of positive controls) for a variant. Criteria also consider population frequency, variant recurrence in tumors, and other evidence. Benign criteria indicating passenger mutation status for the variant are also part of the code set. When all evidence present in literature and databases is reviewed and considered for the variant, then the applicable criteria are applied and summed together to create an overall evaluation of variant oncogenicity. The available annotations are oncogenic, likely oncogenic, variant of unknown significance (VUS), likely benign, and benign.